Table of Contents
ToggleIn the bustling world of animal cells, lysosomes are like the unsung heroes, tirelessly working behind the scenes. These tiny organelles might not have the glitz and glamour of a mitochondrion, but they’re the cleanup crew that keeps the cellular party going. Think of them as the recycling bins of the cell, breaking down waste and ensuring everything runs smoothly. Without them, cells would be knee-deep in their own mess—yikes!
Overview Of Lysosomes
Lysosomes serve vital functions in animal cells, acting as the main site for waste degradation. These membrane-bound organelles contain hydrolytic enzymes that facilitate the breakdown of various biomolecules. Organelles, macromolecules, and cellular debris undergo degradation within lysosomes, ensuring cellular health.
Enzymes within lysosomes operate optimally at low pH levels. This acidic environment is critical for enzyme activity, effectively breaking down complex substances into simpler components. Cells rely on lysosomes to process proteins, lipids, carbohydrates, and nucleic acids, recycling these molecules for future use.
Lysosomal function also plays a significant role in cellular signaling. They contribute to cell signaling pathways that govern responses to stress and nutrient availability. Additionally, lysosomes regulate autophagy, a process where cells disassemble dysfunctional components to maintain homeostasis.
Diseases linked to lysosomal dysfunction highlight their importance. For example, Tay-Sachs disease and Gaucher’s disease stem from enzyme deficiencies, leading to harmful waste accumulation. These conditions underscore the necessity of healthy lysosomal activity for overall cellular function.
Lysosomes form a part of a complex network within the cell. They interact with other organelles, including the endoplasmic reticulum and mitochondria, facilitating communication and coordinated activities. By doing so, lysosomes help maintain the delicate balance of cellular operations.
Through these varied roles, lysosomes support tissue health and contribute to organismal homeostasis. Their ability to recycle and degrade cellular materials showcases their essential place within animal cells. Understanding lysosomes provides valuable insights into cellular processes and the implications of their dysfunction in disease contexts.
Structure Of Lysosomes
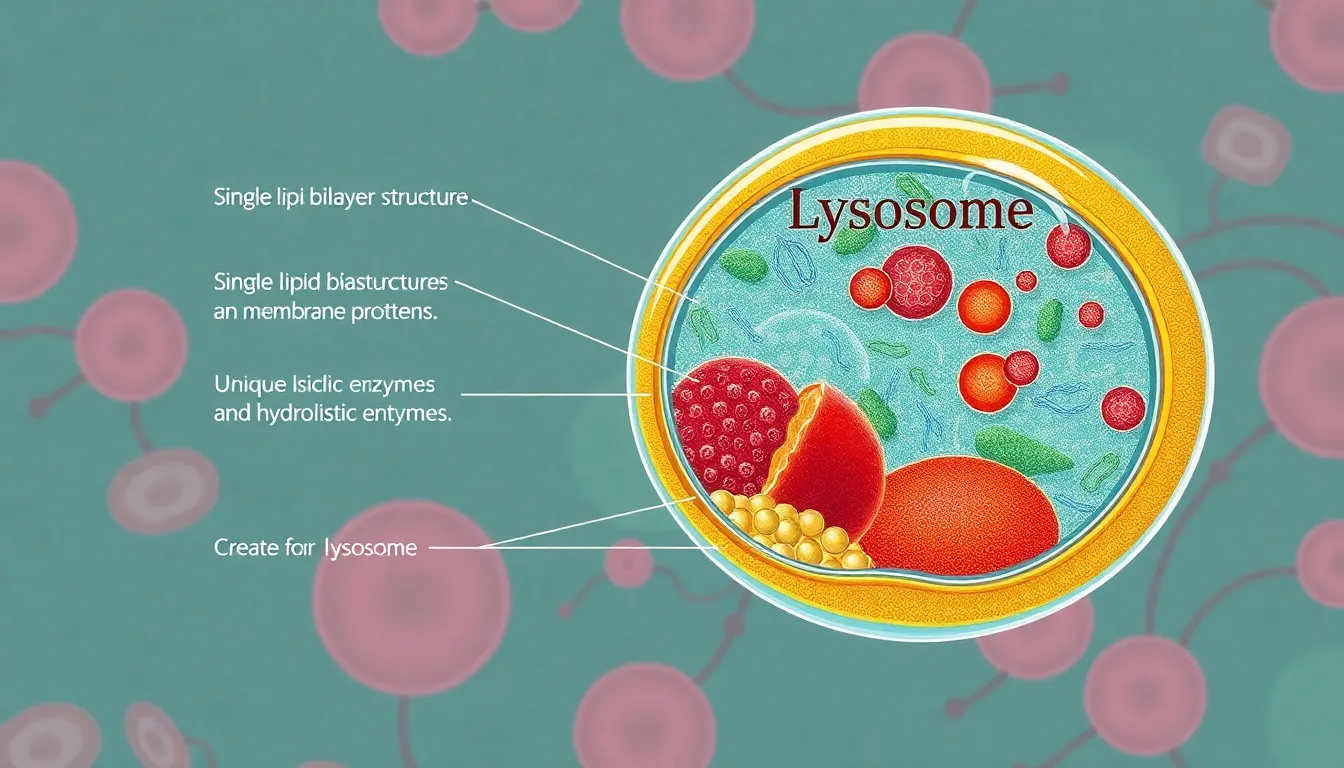
Lysosomes comprise a complex structure critical for cellular function. They feature a single lipid bilayer that encapsulates their contents, providing a protective barrier against the potentially harmful enzymes inside.
Membrane Composition
The membrane of lysosomes contains unique lipids and proteins that distinguish them from other organelles. Phospholipids maintain structural integrity while integral membrane proteins regulate transport. These proteins facilitate the selective exchange of materials, ensuring efficient lysosomal function.
Enzymatic Content
Lysosomes are filled with hydrolytic enzymes essential for digestion and recycling. A wide range of enzymes, including proteases, lipases, and glycosidases, break down biomolecules like proteins, fats, and carbohydrates. Optimal activity occurs at an acidic pH, which is maintained by proton pumps in lysosomal membranes, enabling effective waste degradation.
Lysosome Function In Animal Cell
Lysosomes play critical roles in animal cells, primarily focusing on digestion, autophagy, and metabolism.
Role In Cellular Digestion
Lysosomes act as the primary site for cellular digestion. They contain hydrolytic enzymes that break down waste materials and cellular components. Proteins, lipids, carbohydrates, and nucleic acids undergo degradation within these organelles. As these enzymes function optimally in an acidic environment, lysosomes maintain an appropriate pH through specialized proton pumps. This process ensures that cellular debris and damaged organelles are effectively broken down and recycled, preserving cellular health.
Involvement In Autophagy
Autophagy, a critical process for cellular maintenance, heavily relies on lysosomes. During autophagy, lysosomes engulf and digest obsolete or damaged cellular structures. This mechanism helps cells adapt to stress by recycling components for energy or repairing cellular damage. Increased lysosomal activity during nutrient scarcity highlights their importance in cellular homeostasis. Maintaining balanced autophagy processes prevents cellular accumulation of waste, ensuring efficient function and longevity of cells.
Contribution To Metabolism
Lysosomes significantly contribute to overall cellular metabolism. By breaking down macromolecules, they release essential building blocks and energy sources. This process supports various metabolic pathways, allowing cells to adapt to changing energy demands. Additionally, lysosomes interact with other organelles, including mitochondria and the endoplasmic reticulum, to facilitate nutrient exchange and signaling. Their central role in metabolism underscores the necessity of healthy lysosomal function for maintaining cellular and organismal health.
Disorders Associated With Lysosome Dysfunction
Lysosomal dysfunction leads to various disorders, significantly impacting cellular health. These conditions arise when lysosomes fail to effectively break down waste and recycle biomolecules.
Genetic Lysosomal Storage Diseases
Genetic lysosomal storage diseases result from inherited enzyme deficiencies. Tay-Sachs disease exemplifies this category, caused by a lack of hexosaminidase A, leading to toxic substance accumulation in neurons. Gaucher’s disease, another example, involves glucocerebrosidase deficiency, causing fat buildup in the liver and spleen. Pompe disease occurs due to missing or defective acid alpha-glucosidase, impairing glycogen breakdown in muscle cells. These diseases highlight the critical need for properly functioning lysosomal enzymes to maintain health.
Impacts On Cellular Health
Cellular health relies heavily on lysosome functionality. When lysosomal activity diminishes, waste accumulation increases, resulting in cellular stress and inflammation. Cells may suffer from disruption of metabolic pathways, leading to energy deficits and impaired communication with other organelles. Accumulated waste can result in cell death or dysfunction, affecting tissue and organ function. Overall, lysosomal dysfunction has systemic effects, demonstrating the importance of these organelles in maintaining homeostasis.
Research And Advances In Lysosome Function
Recent studies reveal advancements in understanding lysosome functions. Researchers focus on elucidating their roles in cellular processes such as autophagy and organelle recycling. Techniques like super-resolution microscopy have improved visualization of lysosomes, providing insights into their dynamic behavior within cells.
Novel discovery in lysosomal enzyme mimetics shows potential in treating lysosomal storage diseases. Therapeutic approaches targeting enzyme deficiencies aim to restore normal function, reducing toxic substance accumulation. Moreover, advancements in gene therapy hold promise for addressing genetic causes of lysosomal dysfunction.
Additionally, researchers explore lysosome’s involvement in cellular stress response. These organelles respond to different stressors, helping cells adapt and maintain homeostasis. Findings suggest that lysosomal activity affects metabolic pathways, influencing how cells utilize nutrients and energy.
Studies indicate a connection between lysosomes and neurodegenerative diseases. For instance, impaired lysosomal function has links to Alzheimer’s disease, highlighting the significance of these organelles in neuronal health. Investigating lysosomal roles in neuroinflammation has emerged as a key research area, offering potential therapeutic avenues.
Collaboration between lysosomes and other organelles, such as mitochondria, is under investigation. This interaction plays a crucial role in cellular signaling and energy production. Understanding these relationships enhances the comprehension of cellular homeostasis and reveals new targets for drug development.
Overall, ongoing research continuously uncovers the intricacies of lysosome function. As scientists dive deeper, the therapeutic implications of these findings could revolutionize approaches to treating lysosomal diseases and related conditions.
Lysosomes are indispensable to the health and functionality of animal cells. Their ability to break down waste and recycle cellular components ensures that cells operate efficiently. As research progresses, the understanding of lysosomes continues to deepen, revealing their roles in various cellular processes and diseases.
The link between lysosomal dysfunction and conditions like neurodegenerative diseases underscores the importance of maintaining lysosomal health. Innovations in treatment strategies, including gene therapy and enzyme mimetics, hold promise for addressing lysosomal storage diseases.
Recognizing the multifaceted functions of lysosomes not only enhances our knowledge of cellular biology but also paves the way for potential therapeutic advancements that could significantly impact health outcomes.